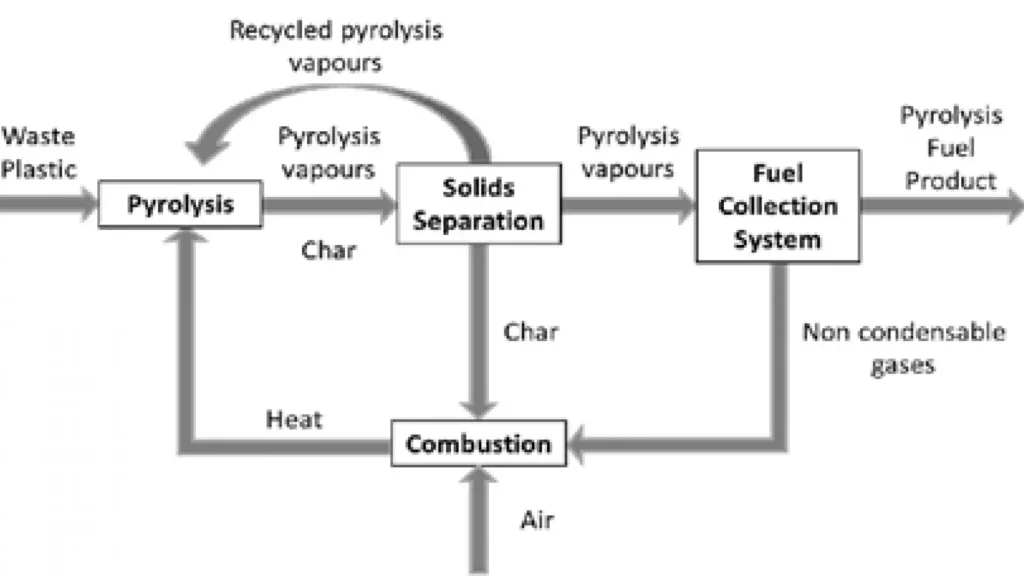
The pyrolysis process occurs in a fluidised bed reactor, in an inert atmosphere, at atmospheric pressure and at a fixed reaction temperature. Thermal cracking of plastics can occur within the temperature range of 450-650 °C, depending on the heating rate and the composition of the plastic waste. The reaction temperature influences directly the pyrolysis product yields.
The pyrolysis vapours include both condensable and non-condensable gases. The condensable gases deliver the oil composed by thermal cracking products. Char is a solid product rich in carbon which can be used to produce heat to the pyrolysis reaction. The non-condensable gas composition depends on the mixture of plastic waste that is pyrolyzed, although some studies say that the main gas components are hydrogen, C1, C2, C3 and C4 hydrocarbons. The pyrolysis thermal energy demand is provided by combusting the pyrolysis by-products (i.e. char, non-condensable gases), no external source of heat is needed and a surplus of heat can be delivered outsite the system. However, electricity demand should be outsourced. If PVC is included in the mix, hydrogen chloride is also produced and it requires chlorine removal before this gas can be used. Figure by Fivga & Dimitriou (2018).
All information in the datasheets is also available in ESDL (Energy System Description Language). You can find them in the Energy Data Repository (EDR).