Nickle-cadmium (NiCd) battery for power applications
Nickle Cadmium (NiCd) batteries store electricity through a reversible chemical reaction. The basic components are a container, electrodes, and an electrolyte. By loading the battery, the electricity is transformed into chemical energy, while during discharge, electrochemical reactions occur at the two electrodes generating a flow of electrons through an external circuit (DNV KEMA, 2013).
The NiCd battery is a mature technology (>100 years) (Chen et al., 2009), however there has been limited commercial success at utility-scale (Luo et al., 2015).
Projects can reach up to 40 MW capacity and typically have discharge times of less than an hour (Chen et al., 2009). The main drawbacks are high costs, toxicity of the materials, and performance degradation due to memory effect (Luo et al., 2015).
Downloads
Download hier de datasheet (PDF)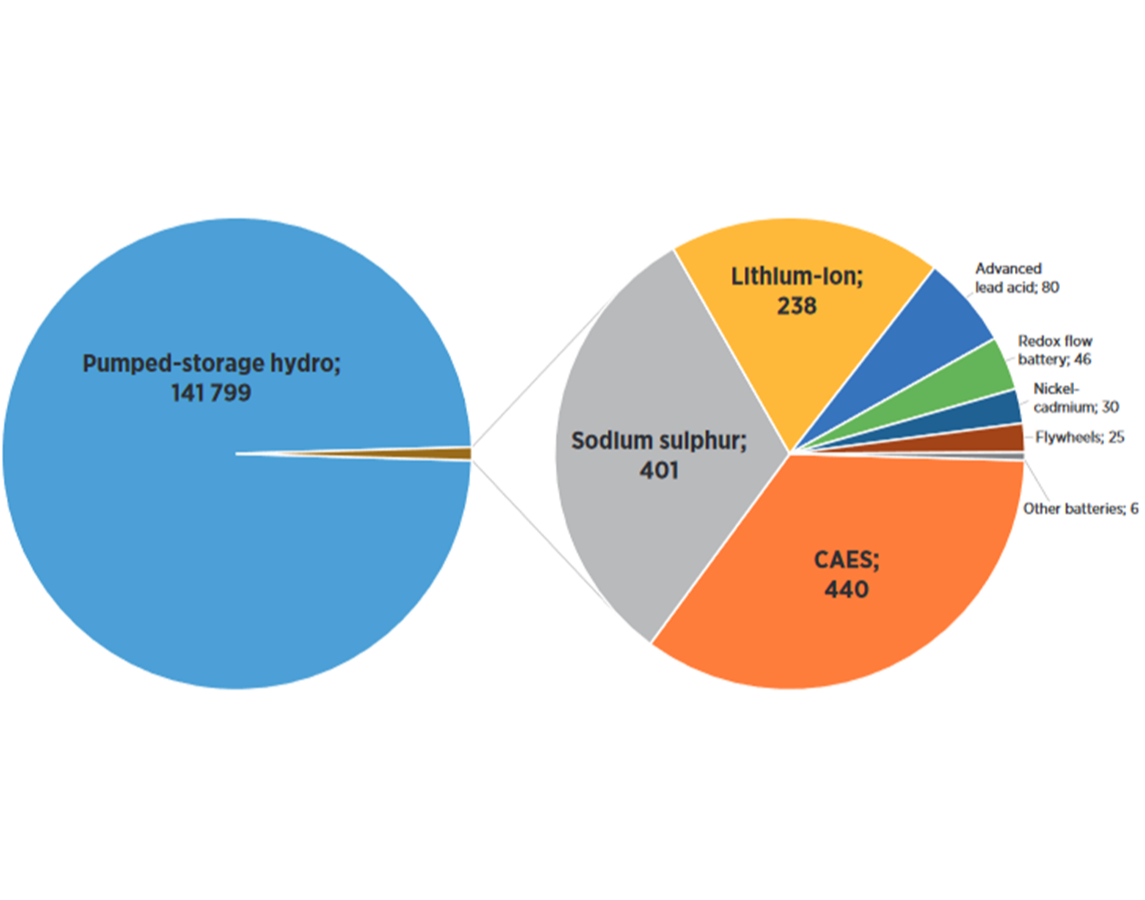
Gerelateerde publicaties

Toename aandeel hernieuwbare warmte
Haalbaarheid van de RED III-doelstellingen met het huidige beleid

Implementatie van energiedelen
Energy sharing: a new activity for active customers and energy communities
Verbruiksprofielen grootverbruikers elektriciteit en aardgas
Twee datasets publiek gemaakt om de vraag van grootverbruikers