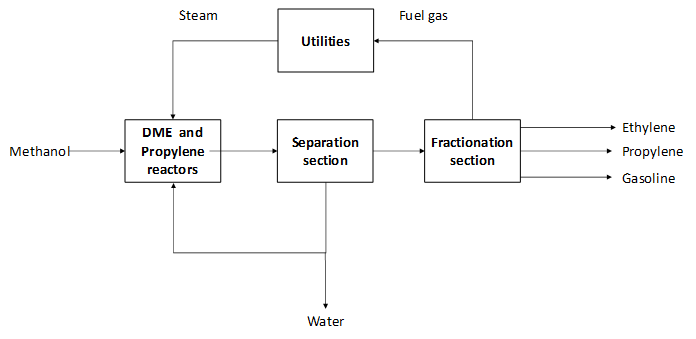
The methanol-to-propylene (MTP) process converts methanol to propylene. Methanol is directed to a first reactor, known as dimethyl ether (DME) reactor, where methanol is converted to DME and water. This stream is fed to another reactor which converts the DME into propylene. This second reaction step takes place on a zeolite-based catalyst (ZSM-5) in a fixed bed reactor, which is different from the methanol-to-olefins (MTO) process. Also, the MTP® process can deliver LPG and gasoline as by-products, which is not the case for the MTO technology. One important element of the MTP® process is the selective catalyst that is able to convert most of the methanol to propylene. Due to coke formation in the second reaction section, this step is normally executed in three reactors that operate in parallel. The coke formation results in carbon losses of less than 5%wt (Rothaemel, M. et al., 2016). One of them is kept in stand-by mode to remove the formed coke by introducing air.
The first step reaction of DME production via methanol can be described by the following reaction:
2 CH3OH → CH3OCH3+ H2O
The second step, the conversion of DME to mainly propylene, is described by the following reaction:
3 CH3OCH3 → 2 C3H6 + 3 H2O
The product stream is directed to the separation section where water is removed and partially recycled to the reaction section and it is used as cooling media. After product conditioning, the product stream is directed to fractionation. There, the product stream is split up into the main product propylene and by-products LPG, ehtylene and gasoline (Jasper, S., El-Halwagi, M. M , 2015 & Zhao, Z. et al., 2020). Polyemer grade propylene (concentration higher than 99.6%wt) can be produced via this technology (Rothaemel, M. et al., 2016).
All information in the datasheets is also available in ESDL (Energy System Description Language). You can find them in the Energy Data Repository (EDR).