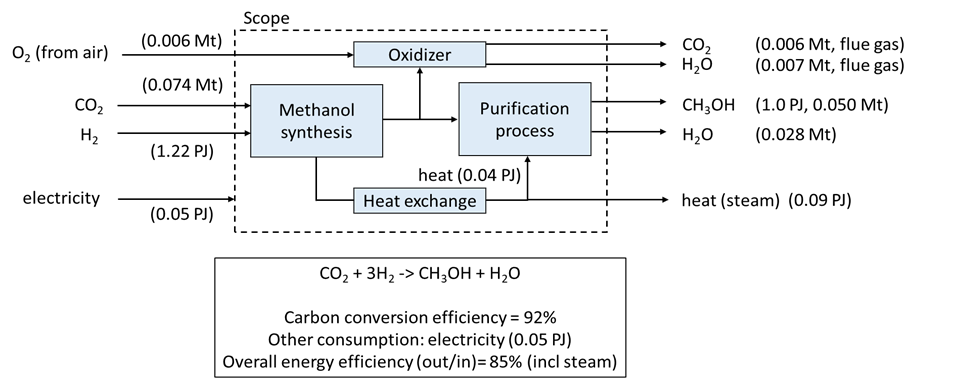
The process runs at a typical temperature of 200-250 °C and at 30-80 bar. The conversion of CO2 in this direct methanol synthesis route is not so high (21%, Anicic, 2014; 33%, Van Dal, 2013), which allows for improvement in the future, e.g. by the development of new catalysts and technologies. After the reaction, the mixture is cooled and led through a flash separator/knock-out drum to separate the gasses from the liquid phase (crude methanol). The majority of the gasses (CO2 and CO) are recycled to the reactor. The crude methanol is purified by leading it through a fractionation column (connected to a heat exchanger) and a stripper unit. The process heat from the synthesis reactor generates steam, which is partly used in the purification process (fractionation and gas stripping) and may be used to generate electricity. Some electricity is required to run the plant, e.g. to drive the compressors for gas compression. The methanol plant is a net electricity consumer and steam producer. Both H2 and CO2 are provided in this case from external sources.
All information in the datasheets is also available in ESDL (Energy System Description Language). You can find them in the Energy Data Repository (EDR).