All information in the datasheets is also available in ESDL (Energy System Description Language). You can find them in the Energy Data Repository (EDR).
Lead-acid (Pb) battery for power applications
Lead-acid batteries store electricity through a reversible chemical reaction. The basic components are a container, electrodes, and an electrolyte. By loading the battery, the electricity is transformed into chemical energy, while during discharge, electrochemical reactions occur at the two electrodes generating a flow of electrons through an external circuit (DNV KEMA, 2013).
Lead-acid batteries can be used for a variety of applications such as bulk storage, frequency regulation, peak shaving, and time-of-use management (IRENA, 2017). This factsheet focuses on power applications (<1h discharge time) such as frequency regulation.
Downloads
Download hier de datasheet (PDF)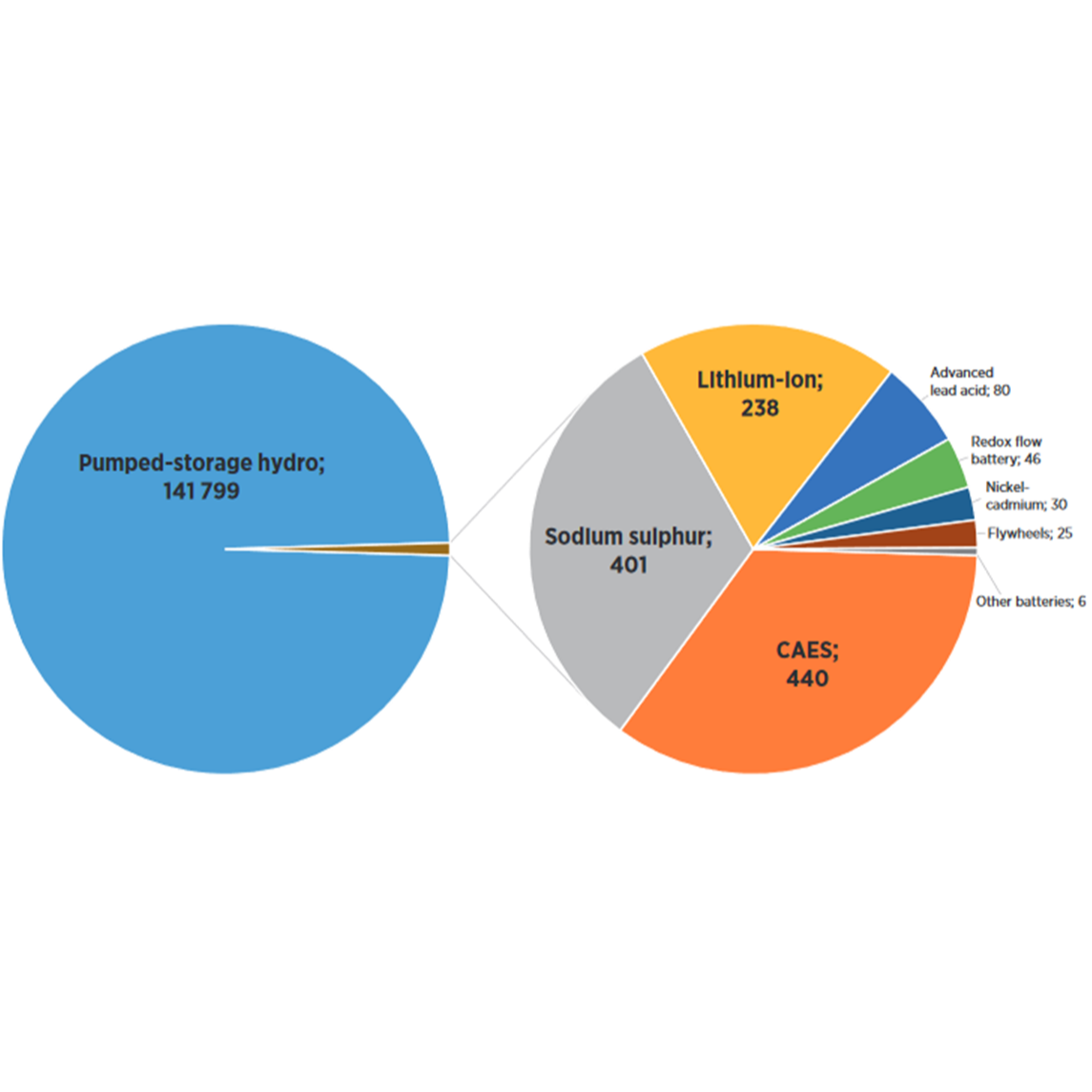
Gerelateerde publicaties

E-trucks: groei en mogelijkheden in de transitie naar elektrisch vervoer
Technische, sociale en organisatorische innovatie nodig voor E-truck-transitie

Toename aandeel hernieuwbare warmte
Haalbaarheid van de RED III-doelstellingen met het huidige beleid

Implementatie van energiedelen
Energy sharing: a new activity for active customers and energy communities