All information in the datasheets is also available in ESDL (Energy System Description Language). You can find them in the Energy Data Repository (EDR).
Lead-acid (Pb) battery for power applications
Lead-acid batteries store electricity through a reversible chemical reaction. The basic components are a container, electrodes, and an electrolyte. By loading the battery, the electricity is transformed into chemical energy, while during discharge, electrochemical reactions occur at the two electrodes generating a flow of electrons through an external circuit (DNV KEMA, 2013).
Lead-acid batteries can be used for a variety of applications such as bulk storage, frequency regulation, peak shaving, and time-of-use management (IRENA, 2017). This factsheet focuses on power applications (<1h discharge time) such as frequency regulation.
Downloads
Download hier de datasheet (PDF)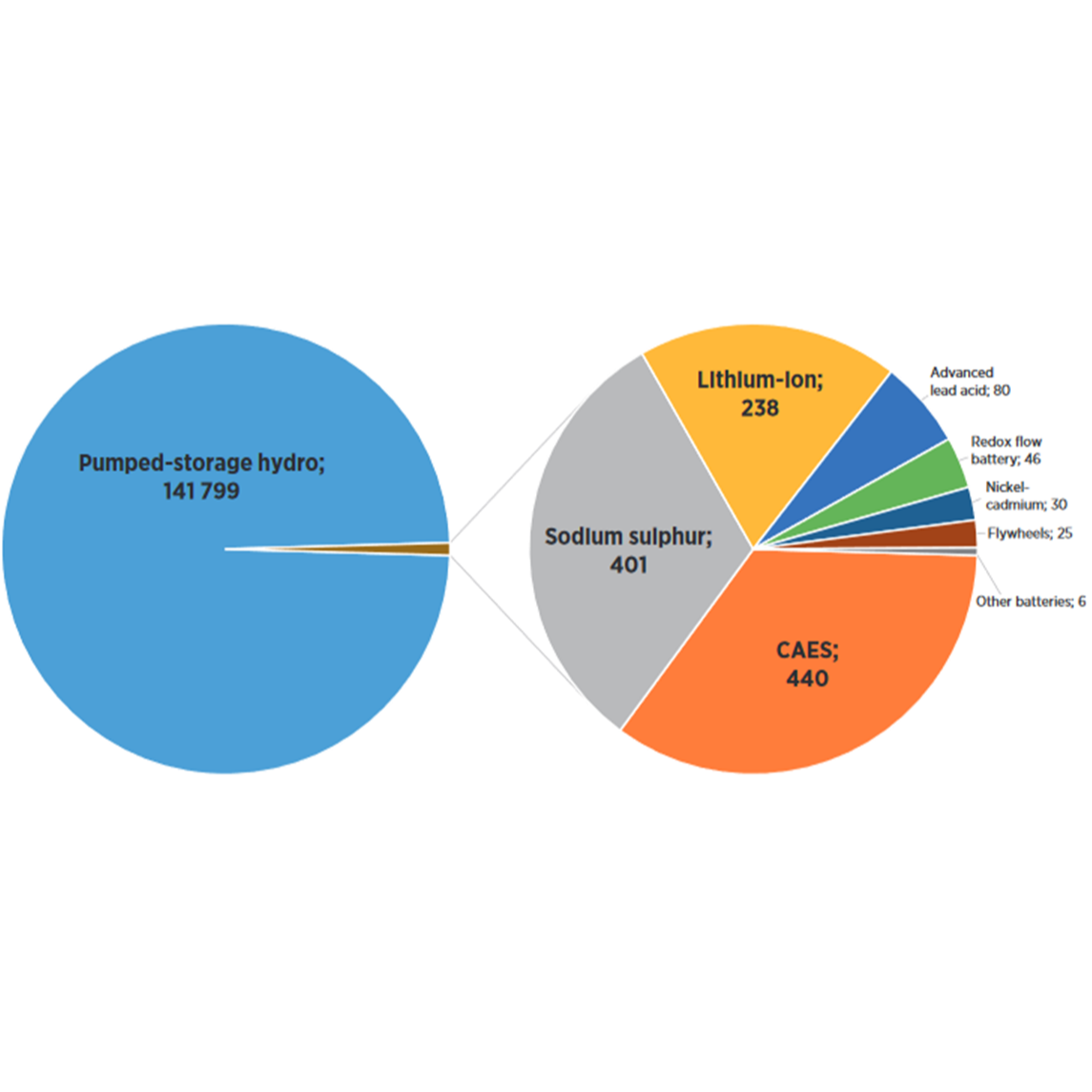
Gerelateerde publicaties

De behoefte aan regelbaar vermogen in 2030-2050
Demand and supply of dispatchable generation in the power system of the Netherlands, 2030-2050

De impact van netcongestie en een wachtrij op de mobiliteitstransitie
Inventarisatie van de gevolgen voor laadinfrastructuur en kleinverbruikers in de Provincie Utrecht

Zet ook de knop om
Verlagen elektriciteitsverbruik huishoudens tijdens piekuren